1/14/2025
Harnessing the Power of Biological Nitrogen Fixation

Crop Insights
Written by Mark Jeschke, Ph.D., Pioneer Agronomy Manager
Key Points
- An adequate supply of plant-available nitrogen is critical to maximizing agricultural productivity.
- The development of industrial nitrogen fixation in the early 20th Century enabled tremendous gains in agricultural productivity, which has been critical to global food security.
- Some of the significant drawbacks associated with synthetic nitrogen fertilizer have led to a renewed interest in leveraging biological nitrogen fixation to provide plant-available nitrogen for crop production.
- Biological nitrogen fixation is carried out by a relatively small subset of bacteria and archaea, known as diazotrophs.
- The nitrogen-fixing organisms most familiar to agriculture are the rhizobia – bacteria that fix nitrogen in root nodules of legumes; however, there are numerous other types of non-symbiotic nitrogen-fixing bacteria.
- Multiple approaches are being explored to increase biological nitrogen fixation in non-legume crops, such as the creation of new types of symbiosis with nitrogen-fixing bacteria and engineering the nitrogen fixation pathway directly into plants.
- Some significant challenges have limited the success of these efforts thus far, including the high energy requirement of nitrogen fixation and the complexity of the nitrogen fixation pathway.
Nitrogen: A Key Building Block for Life
Carbon and nitrogen have some important things in common: both are key building blocks for all life on earth, and both exist in the atmosphere in forms that are not directly usable by living organisms – carbon dioxide (CO2) and dinitrogen (N2). Life on Earth is almost entirely dependent on biochemical processes that have evolved in certain organisms that convert atmospheric carbon and nitrogen into forms that are usable for building organic molecules. These processes are photosynthetic carbon fixation and biological nitrogen fixation.

Figure 1. Legumes like soybean derive a significant portion of the nitrogen needed for their growth from symbiotic associations with nitrogen-fixing Rhizobium bacteria.
Photosynthetic carbon fixation is ubiquitous in nature. There are an estimated 350,000 species on Earth that carry out photosynthesis (Sage and Stata, 2015), including numerous types of bacteria and nearly all plant species. Plants have evolved multiple types of photosynthetic carbon fixation – C3, C4, and CAM – that have allowed plant species to thrive in a wide range of terrestrial environments. The surface layer of the world’s oceans is filled with photosynthetic plankton. While carbon dioxide makes up a relatively small fraction of the atmosphere, life on Earth is very good at accessing and making use of it.
Nitrogen, Nitrogen, Everywhere…
Biological nitrogen fixation, on the other hand, is far less common. While nitrogen exists in great abundance – comprising around 78% of the atmosphere – only a small fraction of living organisms on Earth are able access it. Biological nitrogen fixation is carried out by a relatively small subset of bacteria and archaea, known as diazotrophs. This process is limited almost exclusively to prokaryotes – until very recently, no eukaryotic organisms were known to fix nitrogen. As a result, biologically available nitrogen is the primary limiting factor for all life on Earth.
Overcoming this limitation has been critical in driving agricultural productivity in the 20th and 21st Century. The development of industrial nitrogen fixation in the early 20th Century, known as the Haber-Bosch process, is widely considered one of the most important innovations in human history. Synthetic nitrogen fertilizer was one of the main drivers of the Green Revolution, which saw significant gains in yields of globally important crops, most notably wheat and rice. Synthetic nitrogen fertilizer has enabled tremendous gains in agricultural productivity, which has been critical to global food security; however, it has not been without its drawbacks.
Biologically available nitrogen is the primary limiting factor for all life on Earth.
Problems With Synthetic Nitrogen
One of the biggest drawbacks is the fact that industrial nitrogen fixation uses a lot of energy. The Haber-Bosch process combines dinitrogen with hydrogen under high temperature (750-930°F) and pressure (2,900-3,600 psi) (Gilchrist and Benjamin, 2017). Industrial nitrogen fixation relies heavily on fossil fuels, both for energy to produce the heat and pressure and a source of hydrogen (natural gas) for the reaction. The synthetic nitrogen fertilizer supply chain (including production, transportation, and field use) accounts for 2.1% of global greenhouse gas emissions (Menegat et al., 2022), more than the total amount produced by all aviation globally.
The other main drawback is that much of the nitrogen applied as fertilizer in agricultural production is lost to the environment. Globally, less than half of nitrogen applied to crop land is taken up by the crop (Zhang et al., 2015). Not only is this economically wasteful, the loss of reactive nitrogen from agricultural soils is associated with several adverse environmental consequences, including contamination of ground and surface water, algal blooms in lakes and rivers, hypoxic dead zones in coastal waters, and nitrous oxide emissions into the atmosphere.
Important Terms
- Prokaryote – Single-celled organisms that do not have a nucleus or membrane-bound organelles. Prokaryotes include two domains – bacteria and archaea.
- Eukaryote – Organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, and some unicellular organisms are eukaryotes.
- Diazotroph – Bacteria and archaea that fix atmospheric nitrogen (N2) into bioavailable forms such as ammonia.
- Archaea – Single-celled prokaryotes originally classified as bacteria but now recognized as a separate domain of life. Archaea are often found living in extreme environments.
- Symbiosis – Any type of a close and long-term biological interaction between two organisms of different species. In some cases, such as that of legumes and rhizobia, this relationship can be mutually beneficial.
- Nitrogenase – An enzyme produced by some bacteria and archaea that is responsible for the reduction of nitrogen (N2) to ammonia (NH3).
The Need for Sustainable Alternatives
The drawbacks of synthetic nitrogen fertilizer have led to a renewed interest in leveraging biological nitrogen fixation to provide plant-available nitrogen for crop production. Biological nitrogen fixation is already utilized by crops species such as soybeans, alfalfa, and peanuts that form symbiotic associations with nitrogen-fixing Rhizobium bacteria. The amount of nitrogen fixed through these symbiotic associations can be substantial: grain legumes such as soybeans and peanuts can fix up to 250 lbs of nitrogen per acre and forage legumes like alfalfa and clover can fix 250-500 lbs of nitrogen per acre (Flynn and Idowu, 2015).
Crop scientists have long been interested in developing ways to use biological nitrogen fixation for other crops such as corn, wheat, and rice. Recent advances in plant microbiome research have increased our understanding of additional types of nitrogen fixing bacteria, such as those used in microbial biostimulant products like Utrisha® N (Methylobacterium symbioticum) and Pivot Bio PROVEN®40 (Kosakonia sacchari and Klebsiella variicola). Understanding the potential value of nitrogen-fixing microbial products such as these – both now and in the future – requires a basic understanding of how biological nitrogen fixation works and some of the factors that can enhance or inhibit it.
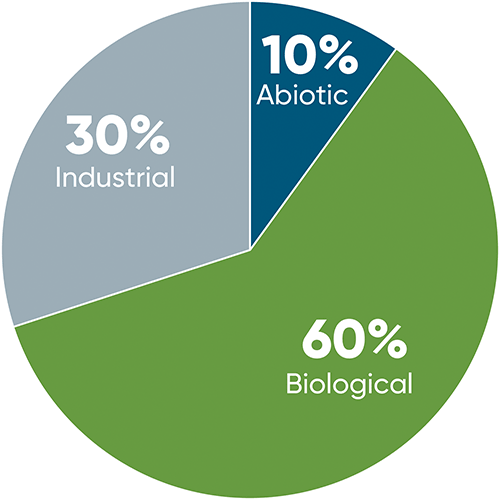
Figure 2. Relative contributions of abiotic, biological, and industrial nitrogen fixation to the total amount of fixed nitrogen on Earth (Hopkins, 1999).
Nitrogen Fixation
The first step to understanding biological nitrogen fixation is understanding what makes atmospheric nitrogen is so difficult to access. It seems counterintuitive that an element that makes up 78% of the atmosphere would also be the element that is most limiting to the growth of living organisms. The answer lies in the basic chemistry of nitrogen atoms. Nitrogen in the atmosphere exists in the dinitrogen form (N2), which is two nitrogen atoms held together by a triple covalent bond. The triple bond between nitrogen atoms is extremely strong, which makes dinitrogen very stable and non-reactive. Breaking this triple bond to make nitrogen available for building useful compounds requires a large input of energy, and there are only a few processes in nature capable of doing this.
Abiotic Nitrogen Fixation
There are three natural abiotic phenomena that provide enough energy to break the dinitrogen triple bond and fix atmospheric nitrogen – fire, ultraviolet radiation, and lightning. All three oxidize atmospheric dinitrogen to nitrogen oxides (NO, N2O). Subsequent reactions then convert these molecules to nitrous acid (HNO2) and nitric acid (HNO3) which can be precipitated out of the atmosphere by rain or snow. Once in the soil, these acids become nitrate (NO3-), which is usable by plants. Lightning is generally thought of as a sporadic occurrence and – in a given location – it is; however, over 3 million lightning bolts strike Earth every day, creating around 13,000 tons of nitrate. Collectively, abiotic nitrogen fixation accounts for around 10% of all nitrogen fixed on Earth (Figure 2).
Biological Nitrogen Fixation
Biological nitrogen fixation is the conversion of dinitrogen to reactive forms by living organisms and is the only other natural nitrogen fixation process aside from fire, lightning, and UV radiation. The protein complex that catalyzes the reaction is called nitrogenase and exists almost exclusively in a small subset of prokaryotes (bacteria and archaea). Biological nitrogen fixation likely evolved early in the history of life on Earth. The first life likely emerged between 3.5 and 4.1 billion years ago and nitrogen isotope ratios in rocks suggest that biological nitrogen fixation has been going on for at least 3.2 billion years (Stüeken et al., 2015). Despite its long history, genomic analysis indicates that only around 5% of bacteria and archaea species have the full set of genes involved in nitrogen fixation (Pi et al., 2022). Biological nitrogen fixation accounts for the majority (~60%) of all nitrogen fixed globally.

Figure 3. The amount of nitrogen fixed through symbiotic associations can be substantial: legumes such as soybeans can fix as much as 250 lbs of nitrogen per acre (Flynn and Idowu, 2015).
Industrial Nitrogen Fixation
The remaining 30% of nitrogen fixation comes from industrial nitrogen fixation via the Haber-Bosch process, which combines dinitrogen from the atmosphere with hydrogen to produce ammonia (NH3). The process was developed by German chemists Fritz Haber and Carl Bosch in the early 20th Century and was first deployed on an industrial scale to produce nitrates for munitions during World War I. Compared to other source of reactive nitrogen, industrial fixation has only existed for a tiny portion of Earth’s history, thus it constitutes an extremely large and abrupt alteration to Earth’s nitrogen cycle. It is estimated that nearly 50% of the nitrogen found in human tissues originated from the Haber–Bosch process (Ritter, 2008).
It is estimated that nearly 50% of the nitrogen found in human tissues originated from the Haber–Bosch process.
Leveraging Biological N Fixation
An adequate supply of plant-available nitrogen is critical to maximizing agricultural productivity. Over the past century, synthetic nitrogen fertilizer produced using the Haber-Bosch process has been a crucial tool for providing that supply of nitrogen. It’s estimated that around half of the world’s current population likely wouldn’t exist if not for this one innovation (Ritchie, 2017). Recent interest in leveraging biological nitrogen fixation to replace a portion of this nitrogen stems from a desire to mitigate the two primary drawbacks of synthetic nitrogen. Biological nitrogen fixation does not rely on fossil fuel inputs for production and, by feeding nitrogen directly to the plant, can greatly reduce or eliminate nitrogen lost to the environment instead of going to produce yield.
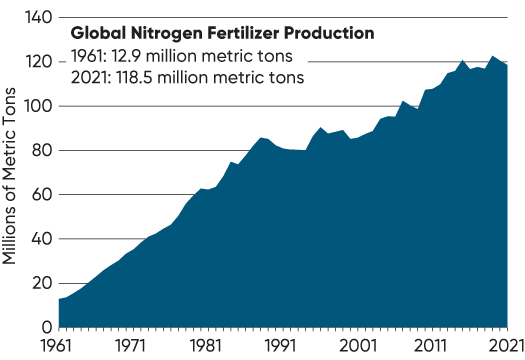
Figure 4. Total global production of nitrogen fertilizer from 1961 to 2021 measured in million metric tons of N (Ritchie et al., 2022).
Breaking Up is Hard to Do
The main challenge to biological nitrogen fixation is the amount of energy required to break the dinitrogen triple bond. Biological nitrogen fixation is one of the most metabolically expensive processes in all of biology, requiring energy from 16 ATP molecules to break one molecule of N2. (As a comparison, C4 photosynthetic carbon fixation costs 5 ATP molecules per molecule of CO2 fixed [Yin and Struik, 2020].) This high energy cost of biological nitrogen fixation is likely a big reason why relatively few species have evolved the capability to do it. It also explains why some plants have developed symbiotic relationships with nitrogen-fixing bacteria. Plant-available nitrogen is a scarce and metabolically expensive resource, so if a plant can partner with bacteria to provide a continual supply, it would be advantageous to do so.
Biological nitrogen fixation is one of the most metabolically expensive processes in all of biology.
The enzyme responsible for catalyzing nitrogen fixation is called nitrogenase, and is the only enzyme known to do this. Four types of nitrogenases are known; three of them are similar and closely related and one is a different enzyme only found in a single bacterial species, Streptomyces thermoautotrophicus. Nitrogenase consists of two component metalloproteins (which is why it is sometimes referred to as an enzyme complex) that together mediate the reduction of dinitrogen (N2) to ammonia (NH3):
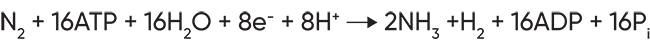
The Black Queen Hypothesis
Biologically available nitrogen is essential for all life on Earth and yet only a small subset of microorganisms are able to produce it – why is this? The Black Queen Hypothesis is a concept that seeks to explain reductive genome evolution and why certain essential functions are rare within some communities (Morris et al., 2012).
“Black Queen” refers to the queen of spades in the game Hearts, where the usual strategy is to avoid taking this card, yet one player must ultimately end up with it. Many vital genetic functions such as nitrogen fixation are “leaky,” meaning that organisms that carry out the function are unable to retain the benefits strictly to themselves. The Black Queen Hypothesis posits that the loss of a metabolically costly and leaky function like nitrogen fixation is selectively favored at the individual level and will proceed until production is just sufficient to support the community.
Simply put – if organisms can attain a costly resource from other organisms instead of producing it themselves, they will. The Black Queen represents the organisms that get stuck carrying the load for the entire community.
Biological Nitrogen Fixation in Agriculture
The nitrogen-fixing organisms most familiar to agriculture are the rhizobia – bacteria in the family Rhizobiaceae that form symbiotic associations with legume species and supply nitrogen to the host plants after becoming established inside nodules on the roots. Legumes include several agriculturally important species such as soybeans, chickpeas, peanuts, lentils, alfalfa, and clover. The supply of nitrogen the legumes receive through these symbiotic relationships allows them to produce seeds high in protein, which has made them important components of both animal and human nutrition. Historically, legumes have been used in agriculture both as a direct source of food and feed, as well as green manure crops that are tilled into the soil to serve as a source of plant-available nitrogen for other crops.
Types of Nitrogen-Fixing Bacteria
The most researched and well-understood diazotrophs are those that function as part of symbiotic systems. In addition to the Rhizobium species, bacteria of the genus Frankia also form symbiotic associations with numerous plants species – mostly trees and shrubs – known as actinorhizal plants. Not all diazotrophs are symbiotic though. Diazotrophs can be grouped into one of three categories based on their life habit (Figure 5).
Free-living: Bacteria that live in the soil and fix nitrogen without direct interaction with other organisms. These bacteria must find their own source of energy, typically by oxidizing organic molecules released by other organisms or from decomposition. Examples include species of Azotobacter, Bacillus, Clostridium, and Klebsiella.
Associative: Bacteria that live near or on plant roots. These bacteria receive energy from the plant via carbohydrates exuded by the roots and fix nitrogen that is usable by the plant but usually do not colonize plant tissues and do not form the same sort of organized interrelationship with a host plant that symbiotic bacteria do. Associative symbiosis is common with grasses such as corn, wheat, rice, and sugarcane and includes genera such as Azospirillum, Glucenobacter, Acetobacter, Herbaspirillum, and Azoarcus.
Symbiotic: Bacteria that colonize the host organism and rely on it for energy while supplying it with fixed nitrogen. The most common examples are root nodule-forming bacteria of the genera Rhizobium and Frankia, but this category also includes nitrogen-fixing cyanobacteria that live in symbiosis with a wide range of terrestrial and aquatic organisms.
Symbiotic diazotrophs account for the majority of total biological nitrogen fixation and are the most important in agricultural systems. Non-symbiotic bacterial diazotrophs have historically had less agronomic significance, but their capability to fix nitrogen is not inconsequential – it is estimated that around 30% of total biological nitrogen fixation comes from non-symbiotic bacteria (Peoples and Craswell, 1992; Kennedy and Islam, 2001).
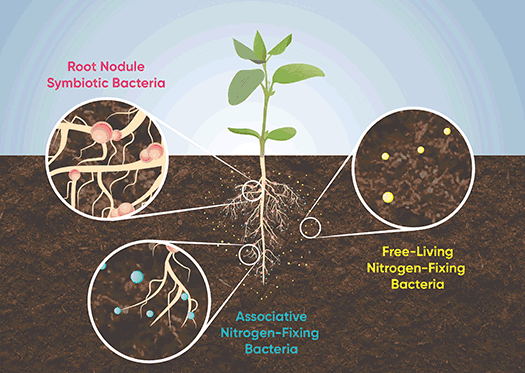
Figure 5. Diazotrophs can be grouped into one of three categories based on their life habit: free-living, associative, or symbiotic.
Looking Beyond Legumes
Enhancing Non-Symbiotic Nitrogen Fixation
One approach to increasing biological nitrogen fixation in non-legume crops is to boost the contribution of free-living and associative nitrogen-fixing bacteria. Numerous genera of nitrogen-fixing bacteria are known to associate with grass crops such as corn and wheat. If the quantity of nitrogen fixed by these bacteria in agricultural systems could be increased, it could reduce the need for synthetic nitrogen fertilizer.
Leveraging non-symbiotic nitrogen-fixing bacteria in crop production is not a new idea. The first commercial inoculant using a free-living species of bacteria was Azotogen (Azotobacter chroococcum), introduced in 1946 (Timonin, 1949). However, several challenges have limited the value and adoption of microbe-based biofertilizers. Some of these challenges have to do with the production, handling, and application of these products – working with living organisms is very different from handling hard chemistries. Other challenges stem from the difficulty in establishing microorganisms in a diverse and dynamic environment where they must compete with other organisms that are already well-adapted to that environment (Roper and Gupta, 2016; Soumare et al., 2020).
Why are Rhizobia So Effective at Fixing Nitrogen?
In exploring ways to enhance non-symbiotic nitrogen-fixation, it’s useful to first look at what makes symbiotic diazotrophs, such as rhizobia, so good at fixing large amounts of nitrogen. Rhizobia colonize the roots of legumes through a highly regulated process of signal exchange between the bacteria and the host plant. When plant-available nitrogen in the soil is limiting, legumes release flavonoids which signal to rhizobia that the plant is seeking symbiotic bacteria. In response, rhizobia release nodulation factors (Nod factors), which stimulate the plant to create deformed root hairs, creating an entry path for the bacteria. Once the rhizobia are inside the root cells, the root cells undergo rapid cell division, forming a nodule.
Nodules on the roots of legumes provide bacteria with two important things that they need for fixing nitrogen: a source of energy and an anaerobic environment.

Figure 6. Nodules that are actively fixing atmospheric nitrogen will be pink or red in color when cut open. The red color is caused by leghemoglobin, a protein that binds to oxygen and makes it available for cellular respiration while preventing it from degrading nitrogenase. Photo from the Manitoba Pulse & Soybean Growers.
The nodule provides the bacteria two important things that they need for fixing nitrogen: a source of energy and an anaerobic environment. Rhizobia are able to fix a lot of nitrogen because the host plants provide them the necessary energy to do it. The energy for splitting the nitrogen gas in the nodule comes from sugar produced by photosynthesis that is translocated from the leaves of the plant. An anaerobic environment is necessary for nitrogenase to function because it is highly sensitive to oxygen, which irreversibly inactivates and degrades the enzyme. The nodule provides the bacteria with this environment. The nodule contains leghemoglobin, an iron-containing protein that binds to oxygen and facilitates its diffusion for use in cellular respiration. Leghemoglobin is what gives the root nodules their characteristic reddish internal color (Figure 6).
Challenges to Non-Symbiotic Nitrogen Fixation
Non-symbiotic diazotrophs do not enjoy the same optimized environment for nitrogen fixation that symbiotic ones do; however, they can still derive some advantages from associating with plants. The plant rhizosphere provides a favorable environment for microbial proliferation compared to bulk soil due to the secretion of root exudates rich in sugars, organic acids, and amino acids, which the bacteria can use as a source of energy. The root exudates of different plants can favor different kinds of bacteria, and several genera are known to associate with corn roots.
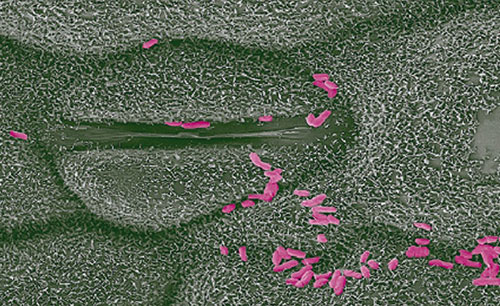
Figure 7. Methylobacterium symbioticum, the active ingredient in Utrisha® N on the surface of a plant leaf. M. symbioticum enter the plant through the stomata and rapidly colonize the entire plant.
The active component in Utrisha® N (Methylobacterium symbioticum) derives energy for nitrogen fixation from its host plant in a different way. Methylobacteria are a genus of bacteria that survive and multiply inside the plant by scavenging methanol, which is a byproduct of normal vegetative plant growth released to the atmosphere through the stomata. The bacteria use methanol as an energy source and fix atmospheric nitrogen. Endophytic bacteria such as M. symbioticum can live and reproduce in plants without causing damage.
Endophytes have an advantage over associative bacteria in the rhizosphere because they can directly access energy from the plant while escaping competition from other organisms. One of the challenges with inoculating soils with bacteria is that the inoculated bacterial populations often decline rapidly due to competition with native organisms (Roper and Gupta, 2016).
Even when non-symbiotic diazotrophs are able to receive energy from a host plant, they generally do not produce large quantities of nitrogen for the plant, simply because they have not evolved to do so. Non-symbiotic diazotrophs typically fix nitrogen for their own needs, not to secrete for uptake by plants (Rosenblueth et al., 2018). These bacteria will reduce nitrogen fixation when it is not to their benefit. Given the high metabolic cost of nitrogen fixation, any bacteria that were investing significant energy into producing excess nitrogen would likely be outcompeted by those that did not. Furthermore, non-symbiotic diazotrophs in the soil typically reduce nitrogen fixation when mineral nitrogen is available, such as in fertilized fields (Knowles, 1980), which can make attempts to replace a portion of applied nitrogen with biological fixation challenging.
Non-symbiotic diazotrophs typically fix nitrogen for their own needs, not to secrete for uptake by plants. These bacteria will reduce nitrogen fixation when it is not to their benefit.
Increasing N Fixation From Bacteria
The levels of nitrogen fixation attained from nitrogen-fixing bacteria in cereal crops such as corn have not been sufficient to support the needs of the crop, so scientists have researched – and continue to research – ways to increase this nitrogen supply.
One strategy has been to increase the amount of nitrogen supplied by associative bacteria. Numerous genera of nitrogen-fixing bacteria are known to associate with the roots of cereal crops such as corn; however, nitrogen supplied by these bacteria is limited by a number of factors, including insufficient colonization and persistence, lack of host specificity, and genetic regulation that limits nitrogen fixation to only what the bacteria need (Haskett et al., 2020). Selecting or modifying bacteria to overcome any one of these limitations could increase the amount available to the crop. Recent research on diazotroph mutants that overproduce and excrete ammonium has shown promise for promoting plant growth (Rosenblueth et al., 2018).
Does Nitrogen Fixation Reduce Yield in Legumes?
Nitrogen fixation is an energy intensive process. Symbiotic bacteria in legumes are able to produce a lot of nitrogen because the energy to carry out the process is supplied by the plant. This raises the obvious question of whether that fixed nitrogen is coming at the expense of productivity. Are the nitrogen-fixing bacteria drawing energy from the plant that could otherwise be going into producing more yield?
The answer is no, not really. Nitrogen fixation definitely has an energy cost for the plant, but so does assimilating nitrate from the soil. Plants need nitrogen in the ammonium (NH4+) form for building amino acids. Rhizobia convert N2 to NH4+, which costs the plant energy. Converting nitrate (NO3-) taken up from the soil to NH4+ also costs the plant energy. The estimated energy costs for these two processes are similar – 605 kJ/mol in the case of nitrate assimilationand 687 kJ/mol for nitrogen fixation (Roesenblueth et al., 2018).
If legumes were able to derive significant advantage from taking up nitrogen from the soil rather than fixing it, experimental evidence would show this, but this has not been the case. Field studies on applying nitrogen fertilizer to soybeans have generally shown small and inconsistent effects on yield (Mourtzinis et al., 2018).
Another strategy has been to engineer new symbioses between non-legume plants and nitrogen-fixing bacteria. This strategy would involve genetically modifying the plant to release nodulation signals to initiate symbiosis and possibly inoculation with a strain of nitrogen-fixing bacteria that would specifically respond to these signals. One of the main challenges to overcome with this approach is the toxic effect of oxygen in the rhizosphere (Haskett et al., 2022; Mus et al., 2016; Soumare et al., 2020). Additional difficulties include efficient delivery of energy to the bacteria and fixed nitrogen to the plant, as well as the sheer complexity of nitrogen-fixing symbiosis, which requires the coordinated function of more than 30 essential genes (Rogers and Oldroyd, 2014).
An important recent discovery is a variety of tropical corn in Mexico that can derive a significant portion of its nitrogen supply from diazotrophic bacteria that live in the mucilage secreted by aerial brace roots. Corn root cap cells secrete a gel called mucilage that contains carbohydrates, amino acids, and other compounds. This gel plays an important role in forming the interface between the root tissue and soil and interactions with soil microbes. Mucilage secreted by brace roots is often visible as droplets that collect at the tips of roots that have not yet reached the soil (Figure 8).

Figure 8. A variety of tropical corn in Mexico was recently discovered that can derive a significant portion of its nitrogen supply from diazotrophic bacteria that live in the mucilage secreted by aerial brace roots.
This variety of corn was cultivated using traditional practices with little or no fertilizer, which may have selected for the evolution of unique microbial associations to provide nitrogen to the plants. It tends to develop multiple nodes of aerial brace roots that produce larger than normal amounts of mucilage. Research found multiple taxa of diazotrophic bacteria in the mucilage that were able to produce enough nitrogen to supply 30-80% of the plant’s needs. The mucilage provided both the energy and the anaerobic environment necessary for nitrogenase to function (Van Deynze et al., 2018).
Engineered N Fixation in Plants
The most direct solution for bringing biological nitrogen fixation to non-legume crops would seem to be to engineer the necessary genes (known as nif genes) into plants so they can do it themselves. After all, engineering bacterial genes into crop plants is something that has already been done, and with great success. Bt corn has been engineered to express a gene from a soil bacterium, Bacillus thuringiensis, that produces a protein highly effective for controlling certain insect pests.
Engineering nitrogen fixation into a non-nitrogen-fixing species has also been done. The successful transfer of nif genes from a nitrogen-fixing bacterium (Klebsiella pneumoniae) to a non-nitrogen-fixing bacterium (Escherichia coli) was achieved all the way back in the early 1970s (Dixon and Postgate, 1972), which made scientists hopeful that this could be achieved with more complex organisms like plants. Creating varieties of agriculturally important crops like corn, wheat, and rice capable of fixing their own nitrogen has long been of interest to scientists and remains an ongoing area of research. However, there are a few challenges that make this a particularly difficult problem to solve and that have – so far, at least – kept this goal out of reach.
Nitrogen Fixation is Complex
The first challenge is that nitrogen fixation is not controlled by just one gene – the nif pathway is large and involves many genes. The nif genes include the genes that code for the two metalloprotein components of nitrogenase, as well as several regulatory proteins involved in nitrogen fixation. Transferring a large gene cluster is difficult enough, but the complexity of the nif pathway makes it particularly challenging. In order to engineer nitrogen fixation into plants, scientists would not only have to transfer the genes, but also replicate the cellular components that control the pathway. Adding to this complexity is the fact that prokaryotes such as bacteria organize their genes in a different way than eukaryotes, such as plants, making the transfer of a multigene cluster into the nuclear genome difficult.
The Oxygen Problem
Nitrogenase is degraded by oxygen – this is an issue that all nitrogen-fixing organisms must deal with but it’s especially problematic for photosynthetic organisms like plants where oxygen is produced as a byproduct of photosynthesis. Overcoming this problem requires some means of compartmentalizing the two processes, either physically or temporally, so they are not going on in the same place at the same time.
There are organisms in nature that can do both – cyanobacteria carry out photosynthesis and many of them also fix nitrogen. One strategy they have evolved to execute both processes successfully is to physically separate the two processes through the development of heterocysts, which are specialized nitrogen-fixing cells. Heterocysts do not engage in photosynthesis and their unique structure allows them to create an anaerobic internal environment favorable for nitrogenase. The heterocysts supply neighboring cells with nitrogen which, in turn, supply them with energy produced through photosynthesis, creating a sort of division of labor among the cyanobacteria. Other cyanobacteria separate the processes temporally, with nitrogen fixation occurring during the night in the absence of oxygen production from photosynthesis (Berman-Frank et al., 2001). So, it is not impossible for photosynthesizing organisms to fix nitrogen, but it comes with some challenges.

Creating varieties of crops like corn capable of fixing nitrogen has long been a dream of plant scientists.
Reasons for Optimism
Although the goal of engineering nitrogen fixation into non-legume crops remains elusive, recent advances in genetic analysis and synthetic biology have enabled significant progress toward this objective. The compilation of a library of thousands of nif gene sequences has given scientists a better understanding of the evolutionary history of nitrogenase (Soumare et al., 2020).
Nodules on the roots of legumes provide bacteria with two important things that they need for fixing nitrogen: a source of energy and an anaerobic environment.
A major new scientific discovery reported in 2024 could provide important insights on a potential path toward nitrogen fixation in plants. Nitrogen fixation was thought to only exist in prokaryotes, but the first example of nitrogen fixation in a eukaryotic organism was recently discovered in a species of unicellular algae (Braarudosphaera bigelowii) (Coale et al., 2024). This species of algae was known to form symbiotic relationships with a nitrogen fixing cyanobacteria called Candidatus Atelocyanobacterium thalassa, or UCYN-A. This type of symbiosis, known as endosymbiosis, involved cyanobacteria living inside the algae cells and exchanging fixed nitrogen for fixed carbon from its host.
Scientists found that this relationship had evolved beyond endosymbiosis to the point where UCYN-A was now functioning as an organelle – its division had come under the control of the host organism, and the symbionts were transmitted to the daughter cells during cell division. Choloroplasts and mitochondria in eukaryotic cells are believed to have evolved in the same way – they were originally separate organisms that became endosymbionts and eventually part of the host organism. This newly discovered nitrogen-fixing organelle was given the name nitroplast. This discovery is a significant development in the pursuit of engineering nitrogen fixation into plants because it shows that nitrogen fixation in a eukaryotic organism is indeed possible.
References
- Berman-Frank, I., P. Lundgren, H. Y.B. Chen, H. Küpper, Z. Kolber, B. Bergman, and P. Falkowski. 2001. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine Cyanobacterium trichodesmium. Science 294:1534-1547.
- Coale, T.H., V. Loconte, K.A. Turk-Kubo, B. Vanslembrouck, W.K.E. Mak, S. Cheung, A. Ekman, J.-H. Chen, K. Hagino, Y. Takano, T. Nishimura, M. Adachi, M. Le Gros, C. Larabell, and J.P Zehr. 2024. Nitrogen-fixing organelle in a marine alga. Science 384:217-222.
- Dixon, R.A., and J.R. Postgate. 1972. Genetic transfer of nitrogen fixation from Klebsiella pneumoniae to Escherichia coli. Nature 237:102–103.
- Flynn, R., and J Idowu. 2015. Nitrogen Fixation by Legumes. Cooperative Extension Service Guide A-129. New Mexico State Univ.
- Gilchrist, M., and N. Benjamin. 2017. From Atmospheric Nitrogen to Bioactive Nitrogen Oxides. In Nitrite and Nitrate in Human Health and Disease, 2nd ed.; Bryan, N.S., Loscalzo, J., Eds.; Humana Press: New York, NY, USA; pp. 11–20.
- Haskett, T.L., A. Tkacz, and P.S. Poole. 2021. Engineering rhizobacteria for sustainable agriculture. The ISME Journal 15:949-964.
- Haskett, T.L., P. Paramasivan, M. D. Mendes, P. Green, B.A. Geddes, H.E. Knights, B. Jorrin, M.H. Ryu, P. Brett, C.A. Voigt, G.E.D. Oldroyd, and P.S. Poole. 2022. Engineered plant control of associative nitrogen fixation. PNAS 119:16.
- Hopkins, W.G. 1999. Introduction to Plant Physiology – Second Edition. pp. 99-121. John Wiley & Sons.
- Kennedy, I.R. and N. Islam. 2001. The current and potential contribution of asymbiotic nitrogen fixation to nitrogen requirements on farms: A review. Aust. J. Exp. Agric. 41:447-457.
- Knowles R. 1980. Methods for evaluating biological nitrogen fixation. pp. 557-582. John Wiley & Sons.
- Menegat, S., A. Ledo, and R. Tirado. 2022. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Scientific Reports. 12:14490.
- Morris, J.J., R.E. Lenski, and E.R. Zinser. 2012. The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio. 3(2):e00036-12.
- Mourtzinis, S., G. Kaur, J.M. Orlowski, C.A. Shapiro, C.D. Lee, C. Wortmann, D. Holshouser, E.D. Nafziger, H. Kandel, J. Niekamp, W.J. Ross, J. Lofton, J. Vonk, K.L. Roozeboom, K.D. Thelen, L.E. Lindsey, M. Staton, S.L. Naeve, S.N. Casteel, W.J. Wiebold, and S.P. Conley. 2018. Soybean response to nitrogen application across the United States: A synthesis-analysis. Field Crops Research 215:74-82.
- Mus, F., M.B. Crook, K. Garcia, A.G. Costas, B.A. Geddes, E.D. Kouri, P. Paramasivan, M.H. Ryu, G.E.D. Oldroyd, P.S. Poole, M.K. Udvardi, C.A. Voigt, J.M. Ané, and J.W. Peters. 2016. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 82:3698 -3710.
- Peoples, M.B., and E.T. Craswell. 1992. Biological nitrogen-fixation—Investments, expectations and actual contributions to agriculture. Plant Soil, 141:13-39.
- Pi, H.W., J.J Lin, C.A. Chen, P.H. Wang, Y.R. Chiang, C.C. Huang, C.C. Young, and W.H. Li. 2022. Origin and evolution of nitrogen fixation in prokaryotes. Molecular Biology and Evolution 39(9), msac181.
- Ritchie, H. 2017. “How many people does synthetic fertilizer feed?” Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/how-many-people-does-synthetic-fertilizer-feed [Online Resource]
- Ritchie, H., M. Roser, and P. Rosado. 2022. “Fertilizers”. Published online at OurWorldInData.org. Retrieved from: ‘https://ourworldindata.org/fertilizers’ [Online Resource]
- Ritter, S.K. 2008. The Haber-Bosch Reaction: An Early Chemical Impact on Sustainability. Chemical & Engineering News. 86 (33).
- Rogers, C., and G.E. Oldroyd. 2014. Synthetic biology approaches to engineering the nitrogen symbiosis in cereals. J. Exp. Bot. 65:1939-1946.
- Roper, M.M., and V.V.S.R. Gupta. 2016. Enhancing non-symbiotic N2 fixation in agriculture. The Open Agriculture Journal 10:7-27.
- Rosenblueth, M., E. Ormeño-Orrillo, A. López-López, M.A. Rogel, B.J. Reyes-Hernández, J.C. Martínez-Romero, P.M. Reddy, and E. Martínez-Romero. 2018. Nitrogen fixation in cereals. Front. Microbiol. 9:1794.
- Sage, R.F., and M. Stata. 2015. Photosynthetic diversity meets biodiversity: The C4 plant example. J of Plant Physiol. 172:104-119
- Soumare, A., A.G. Diedhiou, M. Thuita, M. Hafidi, Y. Ouhdouch, S. Gopalakrishnan, and L. Kouisni. 2020. Exploiting biological nitrogen fixation: a route towards a sustainable agriculture. Plants 9, 1011.
- Stüeken, E.E., R. Buick, B.M. Guy, and M.C. Koehler. 2015. Isotopic evidence for biological nitrogen fixation by molybdenum-nitrogenase from 3.2 Gyr. Nature 520:666-669.
- Timonin, M.I. 1949. Azotobacter preparation (Azotogen) as a fertilizer for cultivated plants. Soil Sci. Soc. Am. J. 13:246.
- Van Deynze, A., P. Zamora, P.-M. Delaux, C. Heitmann, D. Jayaraman S. Rajasekar, D. Graham, J. Maeda, D. Gibson, K.D. Schwartz et al. 2018. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biology 16: e2006352.
- Yin, X., and P.C. Struik. 2021. Exploiting differences in the energy budget among C4 subtypes to improve crop productivity. New Phytol. 229:2400-2409.
- Zhang, X., E.A. Davidson, D.L. Mauzerall, T.D. Searchinger, P. Dumas, and Y. Shen. 2015. Managing nitrogen for sustainable development. Nature 528:51-59.
The foregoing is provided for informational use only. Contact your Pioneer sales professional for information and suggestions specific to your operation. Product performance is variable and subject to any number of environmental, disease, and pest pressures. Individual results may vary. Pioneer® brand products are provided subject to the terms and conditions of purchase which are part of the labeling and purchase documents.