By Bill Mahanna, PhD, Dipl. ACAN, Pioneer Global Nutritional Sciences Manager
One of the silver linings of high grain prices is that it focuses research interest on areas that historically have received limited attention. One such area is the intrinsic physiology and biochemistry of the corn kernel and how specific factors might be quantified or manipulated to improve ruminal and/or intestinal starch digestion.
Kernel Characteristics
The corn kernel evolved fto protect the seed until conditions are suitable for germination. This resulted in a fibrous pericarp protecting both the embryo and its developmental energy source - the starch-rich endosperm. Starch granules are surrounded by hydrophobic proteins that repel water to prevent premature starch hydration that could interfere with germination.
These digestion-resistant seed factors are designed to facilitate seed distribution in the feces of plant-consuming animals and enhance plant reproduction; they were never designed to facilitate digestion.
Proteins surrounding starch granules consist of prolamins, such as zeins, and other proteins (albumins, globulins, glutelins). Prolamins are the starch-encapsulating storage proteins of interest because they are proven to interfere with starch digestion. They derive their name from a relatively high content of the amino acid proline (and glutamine).
Corn prolamins tend to be in higher concentrations in the vitreous (glassy) endosperm than in the more centrally located floury endosperm. Prolamins for each cereal grain have specific and historic names (wheat - gliadins, oats - avenins, barley - hordeins), and small grains have a lower prolamin content than corn (zeins) or sorghum (kafirins; Hoffman and Shaver, 2008).
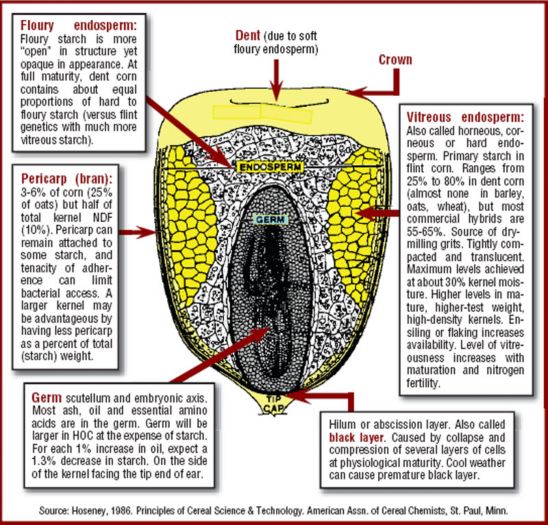
Based on kernel characteristics, corn grain can be divided into five types: flint, popcorn, floury, dent and sweet corn (Corona et al., 2006). The endosperm of flint corn consists of hard-textured, densely packed, crystalline starch that is also called corneous, horny, translucent or vitreous starch. The endosperm of floury corn is soft textured, and when mature and dry, weakness of the discontinuous protein matrix allows starch granules to separate. Air space between starch granules results in a lower absolute (water or gas displacement) density for grain with more floury than vitreous starch (Szasz et al., 2006).
Yellow dent corn, common in North America, is the result of historic crossbreeding between flint and floury types. The name dent is derived from the indentation at the top of the kernel as the floury internal starch shrinks during kernel maturation and drying.
Shorter-season hybrids tend to have more flint in their pedigree that increases cold tolerance and early vigor of the seedling. Hybrids with more floury genetics often have a lower absolute density and lower test weight, but only about 40% of the variation in grain density can be attributed to the variation in test weights due to influences of kernel size, shape, maturity, germ content and pericarp slickness (Szasz et al., 2006; Owens, 2009).
Yellow dent hybrids with more vitreous endosperm also have a higher prolamin content compared with lower-density dent hybrids. Studies using mature grain from inbred lines that were grown across locations and years show that genetics, rather than the growing environment, can account for more than 93% of the variation in alcohol-soluble storage proteins (Smith and Smith, 1986).
Interest in corn kernel proteins dates back to 1892 and has been driven primarily by food quality and processing issues. The term zein that identifies the class of corn prolamins was introduced by professor Gorham of Harvard University in 1821 (Sherman and Winters, 1918). Four types of zein - alpha, beta, gamma and delta - are found encapsulating the surface of corn starch granules. Concentrations of prolamins increase with kernel maturation and peak at the time of black layer formation (about 30% kernel moisture). Alpha and beta zein penetrate the endosperm, whereas beta and gamma cross-link to form the hydrophobic “starch/protein” matrix (Hoffman and Shaver, 2008).
Zein differs from other kernel proteins in that it is soluble only in aqueous alcohol or fermentation acids (lactic, acetic) but not in water (or rumen fluid). Microbial activity during fermentation and the chemical action of various fermentation end products (acids, yeast-generated alcohol) as well as gelatinization (starch damage associated with heat processing) serve to alter kernel storage proteins, removing most of the negative effects of zeins on starch digestibility. Consequently, total tract starch digestibility typically exceeds 96% for adequately fermented/processed (silage or high-moisture grain) and steamflaked (or steam-rolled) corn (Owens and Soderlund, 2007; Firkins, 2006).
Starch Digestion Primer
Starch granules are composed of two different polymers. Amylose (alpha [1-4] linked glucose) forms linear chains and comprises about 24-30% of the starch in normal dentf corn, while amylopectin (alpha [1-4] glucose linkages with alpha [1-6] branches about every 20 glucose units) contributes to the crystalline structure that encases the more amorphous lamellae.
Digestibility of starch by non-ruminants is related to the degree of branching (amylose:amylopectin ratio), with higher amylose (less branching) content and the presence of more “resistant” starch depressing that rate and extent of the starch digestion (Owens, 2005b; Svihus et al., 2005).
For the ruminant’s microflora, starch digestibility is influenced to a greater degree by accessibility (presence of pericarp, endosperm protein-matrices) of starch granules than by amylose content, perhaps because the particle size of processed grain is typically larger for grain fed to ruminants than to nonruminants (Owens, 2009).
In the rumen environment, once the pericarp is breached, accessibility of starch granules remains limited by the protein matrix and endosperm cell walls. The lipid:starch complexes found in the endosperm retard digestion by reducing contact between the digestive enzymes and substrate.
Lipid:starch complexes (higher in smaller granules) also can inhibit starch granule swelling by increasing hydrophobicity. This impairs starch digestion because water is needed for enzymatic degradation. Inhibiting water uptake may also lower the extent of gelatinization during flaking by reducing the extent of granule swelling and abrasion of the endosperm crystalline structure (Svihus et al., 2005).
Most ruminal bacteria that can ferment starch lack beta-glucanases and cannot breach the endosperm cell walls. These organisms depend on cellulolytic organisms to penetrate physical barriers to provide access to starch granules (McAllister et al., 2001). Compared to coarse grinding, fine grinding increases the rate of starch digestibility to a limited degree by disrupting endosperm cell walls.
The protein matrix of corn and sorghum grain resists proteolytic attack and restricts access of encased starch granules to bacterial amylases. In cereals like barley, starch digestibility is rapid due to lower prolamin (hordeins) concentrations and weaker associations, allowing for easier degradation by a variety of proteolytic bacteria.
Unlike the endosperm of corn and sorghum, the endosperm of barley is homogeneous, and starch granules are loosely packed within the protein matrix (Svihus et al., 2005).
The stronger protein:starch bond in corn and sorghum may explain why processing these two grains results in a large number of cracked starch granules, greater surface area and increased ruminal degradation compared to cereals like barley or wheat (Svihus et al., 2005). To bacteria, a starch granule is a starch granule. McAllister et al. (1993) demonstrated that when freed from the protein matrix, starch granules from both barley and corn are digested at similar rates.
The bacteria in the rumen primarily involved with starch digestion are Streptococcus bovis, Ruminobacter amylophilus, Prevotella ruminicola, Butyrivibro fibrisolvens, Succinomonas amylolytica and Selenomonas ruminantium. Each of these bacteria can digest starch, but none is capable of producing the variety of enzymes needed to digest the entire grain kernel. Thus, bacterial access to starch granules clearly requires symbiosis among bacteria to remove impediments to digestion.
Starch digestion is initiated by complementary bacterial species that form a complex digestive “consortium” at the exposed surface of the grain. Initially, amylolytic bacteria are attracted and adhere to starch granule surfaces. They multiply and produce digestive enzymes that release soluble nutrients through the formation of “digestive pits” on the surface of starch granules. This attracts secondary colonizers to the digestive site.
In time, the entire surface of the starch granule becomes coated with a multi-species amylolytic and proteolytic population. Factors that alter this sequential development, such as grain processing, can profoundly affect both the rate and extent of cereal grain digestion in the rumen (McAllister et al., 2001).
Two groups of protozoa (Holotrichs and Entodiniomorphs) also degrade starch after they engulf starch granules. Consumption rates are inversely related to the size of starch granules. Protozoa contribute 20-45% of the total amylolytic activity in the rumen. The population of rumen protozoa is reasonably high even with grain-based finishing diets, and the role of protozoa in starch digestion, particularly at its early stages, probably is greater than indicated by early studies (McAllister et al., 2001).
By engulfing starch granules for more gradual digestion relative to the more explosive fermentation of ruminal bacteria, protozoa retard the rate of starch digestion and moderate the drop in ruminal pH post-feeding. Protozoa have more prolonged digestion, with 36 hours needed to fully metabolize engulfed granules.
Being attached to, or associated with, particulate matter of the rumen, protozoal outfl ow from the rumen is slower than for bacteria. The abrupt change in acid and osmotic pressure experienced by protozoa leaving the rumen causes them to “explode,” exposing engulfed starch for digestion by intestinal enzymes.
Protozoa also are predatory, engulfing amylolytic bacteria to reduce their population, which, in turn, can reduce the rate of fermentation acid production. Certain fungi (e.g.,Neocallimastix frontalis) also produce amylolytic enzymes, and all fungi have hyphae that exert physical forces to help penetrate recalcitrant plant structures such as the grain pericarp (McAllister et al., 2001).
Starch that escapes fermentation in the rumen is attacked by pancreatic amylases in the small intestine. Grain processing methods that increase ruminal degradation of starch generally increase the digestibility of residual starch that enters the intestines (Svihus et al., 2005), although it is a reduced supply due to more extensive digestion of starch in the rumen.
Starch absorbed as glucose from the intestines can have more than 20% greater caloric value than starch fermented to volatile fatty acids within the rumen, so some sacrifice of small intestinal digestibility can be tolerated. Shifting the site of digestion downstream also will reduce the likelihood of ruminal acidosis. However, reducing the supply of fermentable energy for ruminal bacteria will reduce microbial yield from the rumen.
If the metabolizable protein supply is low or marginal, milk production could be depressed. If metabolizable protein needs are being adequately met by the current diet, increasing the extent of ruminal starch digestion in order to enhance the supply of microbial protein is unlikely to prove beneficial. However, in some studies, an increased flow of protein to the intestines has increased dry matter intake (Owens, 2009).
The proportion of total starch digested or fermented post-ruminally is much greater for lactating cows than for finishing steers, presumably due to higher feed and neutral detergent fi ber intakes that decrease the time starch particles will remain in the rumen for microbial fermentation (Owens and Soderlund, 2007).
Quantifying Storage Proteins
Yale biochemist Thomas Burr Osborne, supported by some of the first funding of the Hatch Act, published work in 1897 that described corn storage proteins, including the alcohol-soluble prolamins, the zeins (Sherman and Winters, 1918). Nearly 75 years later, laboratory procedures were published that outlined methods to isolate and separate the various corn protein fractions (Landry and Moureaux, 1970).
Due to differences in amino acid composition of protein types, protein fractionation became a key focus for human nutritionists. Parallel interest was not shared by dairy nutritionists or agricultural labs likely because Goering and VanSoest published the detergent system of fiber analysis in the same year (Hoffman and Shaver, 2008).
Furthermore, these corn protein fractionation methods were arduous and detailed, and only the hydrophobic prolamins appeared to be negatively associated with starch digestibility (Hoffman and Shaver, 2008, Philippeau et al., 2000). It seems surprising that similar protein fractionation procedures have not been employed for the prediction of ruminal escape of dietary protein and amino acids from various protein sources and feedstuffs (Owens, 2009).
Recently, Larson and Hoffman (2008) at the University of Wisconsin’s Marshfield Agricultural Research Station published a turbidimetric procedure for zeins (mTZM) based on cereal chemistry techniques and rapid turbidimetric methods. This procedure simplifies the quantification of total zeins in dry and high-moisture corn kernels. Corn kernels from very diverse flint, dent, floury and opaque endosperm types were profiled and were found to contain 19.3, 11.3, 5.9 and 4.9 g of zeins per 100 g of starch, respectively.
Commercialization of the mTZM method should increase our understanding of the range in prolamin content among commercial corn grain that is produced by differing genetics under a wide range of geographies and growing conditions. As of the writing of this column, mTZM is still under development, is being evaluated for repeatability between laboratories and is not yet commercially available. However, developers hope the test will be commercialized within the next year at an estimated cost of less than $15 per sample (Hoffman, 2009).
The development of mTZM is a very positive analytical advancement that should help move grain evaluation beyond semi-quantitative measurements, like test weight and vitreousness, to better-defined chemical constituents that may have a more direct impact on digestibility.
However, the utility of measuring total prolamins needs to be validated by studies with ruminants fed rations containing grain from commercially productive hybrids expressing a typical range of prolamin content.
Such research will help determine how large a difference in grain prolamin content is necessary to affect realworld rations and help prioritize the relative importance of harvest moisture, kernel starch content, grain processing methods, particle size and vitreousness (prolamin content) on animal performance.
Future investigation of other chemical factors related to cereal grains should help quantify the effects of not only specific prolamin subtypes but also dehydrin content (proteins required for desiccation tolerance as the kernel matures and dries) and of kernel lipids on starch digestibility by ruminants (Hoffman, 2009; Svihus et al., 2005).
The Bottom Line
The economic and ruminal health implications of starch digestibility are immense. Digestion of corn grain by dairy cattle appears to be limited primarily by large particle size and the pericarp shielding the endosperm. In addition, the starch fermentation rate can be reduced in mature dry corn depending on the level of protein shielding (presence of prolamin proteins, commonly called zeins) that surrounds and links the starch granules in the portion of the endosperm containing more dense, vitreous starch.
Fine grinding will increase starch digestion by cattle, but more extensive processing methods such as ensiling or flaking greatly minimize or obliterate the effect of vitreousness on starch digestion. In addition to increasing total tract digestion, extensive processing also can shift the site of starch digestion.
Concerns about a slight reduction in ruminal starch digestibility have driven researchers in Europe and South America (where flinty hybrids are more common) and, more recently, in North America to examine the effects of vitreousness (and zein content) on starch digestibility of corn grain samples that differ in vitreousness (absolute density). It has also spurred development of a new lab test, mTZM, to better quantify the corn zeins (prolamins) that slow starch digestion.
Assuming that measuring prolamin content is repeatable in the lab, in vivo animal studies using real-world rations are needed to assess corn prolamin effects on milk production and to rank cereal chemistry parameters in relation to other factors known to affect starch digestibility, such as kernel moisture/ maturity, kernel size, starch content, grain processing method and grain particle size/distribution.
The March 9 Bottom Line column will be a continuation of this discussion of starch digestibility that delves into the modifying effect of processing methods like fermentation and flaking, issues surrounding research conclusions that can draw from the current body of published literature and considerations to keep in mind when selecting corn hybrids.
References
- Corona, L., F.N. Owens and R.A. Zinn. 2006. Impact of corn vitreousness and processing on site and extent of digestion by feedlot cattle. J. Anim. Sci. 84:3020-3031.
- Firkins, J.L. 2006. Starch digestibility of corn – Silage and grain. Proc. Tri-State Dairy Nutrition Conference. April 25-26. Hoffman, P.C. 2009. Personal communication.
- Hoffman, P.C., and R.D. Shaver. 2008. Corn biochemistry: Employing cereal chemistry techniques in grain and corn silage analysis. 2008 Penn State Dairy Cattle Nutrition Workshop. Grantville, Pa. Nov. 12-13.
- Landry, J., and T. Moureaux. 1970. Heterogenicity of the glutelins of the grain core. Selective extraction and composition in amino acids of the three isolated fractions. Bull. Soc. Chem. Bio. 52:1021-1037.
- Larson, J., and P.C. Hoffman. 2008. Technical note: A method to quantify prolamin proteins in corn that are negatively related to starch digestibility in ruminants. J. Dairy Sci. 91:4834-4839.
- McAllister, T., A. Hristov and Y. Wang. 2001. Recent advances/current understanding of factors impacting barley utilization by ruminants. Proc. 36th Annual Pacific Northwest Animal Nutrition Conference, Boise, Ida. Oct. 9-11.
- McAllister, T.A., R.C. Philloppe, L.M. Rode and K.J. Cheng. 1993. Effect of the protein matrix on the digestion of cereal grains by ruminal microorganisms. J. Animal Sci. 71:205-212.
- Owens, F.N. 2005. Corn grain processing and digestion. Proc. 66th Minnesota Nutrition Conference. Sept. 20-21. St. Paul, Minn.
- Owens, F.N. 2009. Personal communication. Pioneer senior research scientist.
- Owens, F., and S. Soderlund. 2007. Getting the most out of your dry and high-moisture corn. Proc. 4-State Dairy Nutrition & Management Conference. Dubuque, Iowa. June 14.
- Philippeau, C., J. Landry and B. Michalet- Doreau. 2000. Influence of the protein distribution of maize endosperm on ruminal starch digestibility. J. Sci. Food & Agric. 80:404-408.
- Sherman, H.C., and J.C. Winters. 1918. Efficiency of maize protein in adult human nutrition. J. Biological Chemistry.
- Smith, J.S.C., and O.S. Smith. 1986. Environmental effects on zein chromatograms of maize inbred lines revealed by reversedphase high-performance liquid chromatography. Theor. Appl. Genet. 71:607-612.
- Svihus, B., A.K. Uhlen and O.M. Harstad. 2005. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 122:303-320.
- Szasz, J., C. Hunt and F. Owens. 2006. Impact of starch source and processing on feedlot production with emphasis on high-moisture corn. 2006 Pacific Northwest Nutrition Conference Proceedings.
This article was originally published in February 2009 Feedstuffs issue, and is reproduced with their permission.